Inhaltliche Hinweise
allgemein
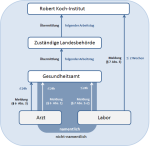
 Melde- und Übermittlungswege gemäß Infektionsschutzgesetz (IfSG)
Quelle: RKI
Melde- und Übermittlungswege gemäß Infektionsschutzgesetz (IfSG)
Quelle: RKI
-
Meldeweg
Bundesweite Meldepflichten sind im Infektionsschutzgesetz (IfSG) festgelegt. Die Meldungen erfolgen über verschiedene Meldewege: die namentliche oder nichtnamentliche Meldung an das Gesundheitsamt oder die nichtnamentliche Meldung direkt an das Robert Koch-Institut (RKI):
-
Die gemäß § 6 Abs. 1 Nr.1 IfSG meldepflichtigen Krankheiten und gemäß § 7 Abs. 1 IfSG meldepflichtigen Nachweise von Krankheitserregern werden namentlich an das zuständige Gesundheitsamt gemeldet. Das Gesundheitsamt stellt nach Meldung eines Falles zusätzlich eigene Ermittlungen an. Dadurch können weitere Fälle im Umkreis des Erkrankten identifiziert werden, die ebenso in die Meldedaten eingehen. Erfüllt ein Fall die vom RKI erstellte Falldefinition, wird er in anonymisierter Form an die zuständige Landesbehörde und von dort weiter an das RKI übermittelt.
-
Besteht eine Meldepflicht gemäß § 7 Abs. 3 IfSG, erfolgt die Meldung nichtnamentlich direkt an das RKI. Für diese Fälle existieren keine Falldefinitionen. Die Fallprüfung erfolgt stattdessen im RKI anhand festgelegter Fallkriterien. Das RKI wertet die über beide Meldewege eingehenden Daten infektionsepidemiologisch aus und veröffentlicht diese periodisch.
-
Die bundesweite Meldepflicht gemäß IfSG kann in den Bundesländern durch Landesverordnungen ausgeweitet werden. Von dieser Möglichkeit haben Bayern, Berlin, Brandenburg, Hessen, Mecklenburg-Vorpommern, Rheinland-Pfalz, das Saarland, Sachsen, Sachsen-Anhalt und Thüringen Gebrauch gemacht. Im Epidemiologischen Bulletin 5/2009 wurden Falldefinitionen für einige dieser zusätzlich meldepflichtigen Krankheiten und Erregernachweise veröffentlicht. Diese sind in SurvStat@RKI 2.0 nach Auswahl des Meldewegs ‚über Gesundheitsamt und Landesstelle‘ und Setzen des Merkmals Meldepflicht auf den Wert: ‚gemäß länderspezifischer Meldeverordnung‘ ebenfalls abfragbar.
-
Die unter SurvStat@RKI 2.0 angebotenen Daten werden wöchentlich (mittwochs) zum Stichtag der Erstellung der neuesten Ausgabe des Epidemiologischen Bulletins aktualisiert. Gemäß § 6 Abs. 1 Nr. 1 und § 7 Abs. 1 IfSG erhobene Daten werden dort wöchentlich publiziert. Daten die sich (gemäß § 7 Abs. 3 IfSG) aus einer direkten Meldung an das RKI ergeben, werden monatlich aktualisiert. Der Datenstand unterscheidet sich also für die beiden Meldewege und wird daher getrennt ausgewiesen.
-
In SurvStat@RKI 2.0 können, für alle Krankheiten und Erregernachweise, deren Meldung eine nähere Spezifikation des Erregers vorsieht, Erregerangaben abgefragt werden. Durch Zusammenfassung einzelner Erregersubtypen wird eine reduzierte/vereinfachte Erregerauswahl angeboten.
-
Ortsangaben. Namentlich zu meldende Krankheiten und Erregernachweise werden örtlich dem Kreis des zuständigen Gesundheitsamtes zugeordnet, was meist dem Hauptwohnsitz des Patienten entspricht. Für nichtnamentlich direkt an das RKI zu meldende Erregernachweise erfolgt die Meldung und Zuordnung des Patientenwohnorts anhand der ersten drei Ziffern der fünfstelligen Postleitzahl. Die Bundesländer stellen die gröbste Gliederungsebene für Ortsangaben in SurvStat@RKI 2.0 dar. Etwas feiner differenzieren die sogenannten Gebietseinheiten, die die territoriale Gliederung Deutschlands gemäß der europäischen „Systematik der Gebietseinheiten für die Statistik“ NUTS wiedergeben. Die Gebietseinheiten entsprechen weitgehend der Aufteilung in die, in den meisten Bundesländern nicht mehr existenten, Regierungsbezirke. Auf der feinsten in SurvStat@RKI 2.0 dargestellten territorialen Gliederungsebene, bei der Ortsangabe nach Kreis, stehen die Abkürzungen LK und SK für Landkreis und kreisfreie Städte.
Für Erregernachweise, die gemäß § 7 Abs. 3 IfSG gemeldet werden, liegen keine Ortsangaben auf Kreisebene vor. Als kleinste strukturelle Einheit wird hier die sogenannte Region angeboten. Es handelt sich dabei um eine Unterteilung der Gebietseinheit in Großstädte und den übrigen, nicht-großstädtischen Anteil.
Bei der Auswertung und Interpretation der Daten nach Ortsangabe ist zu beachten, dass der Wohnort des Patienten nicht notwendigerweise dem Infektionsort entspricht. Die Infektion kann auch in einem anderen Kreis/Bundesland oder im Ausland erworben worden sein. Angaben zum Infektionsort sind in SurvStat@RKI 2.0 nicht abfragbar.
-
Für den regionalen Vergleich der Häufigkeit einer Krankheit (z.B. zwischen Kreisen oder Bundesländern) sollte anstelle der absoluten Fallzahlen die Inzidenz (Anzahl der Fälle pro 100.000 Einwohner) betrachtet werden, um Unterschiede in den Bevölkerungszahlen zu berücksichtigen. Dies gilt auch bei alters- und geschlechtsspezifischen Vergleichen. Beachten Sie, dass, bei insgesamt niedrigen Fallzahlen, für Kreise bzw. Altersgruppen mit niedriger Bevölkerungszahl Abweichungen von nur 1 oder 2 Fällen, in der Inzidenzdarstellung einen großen Unterschied vortäuschen können.
Inzidenzen <0,01 werden immer auf 0,00 gerundet angegeben.
-
Abweichend von früheren Versionen werden in SurvStat@RKI 2.0 keine durchschnittlichen Jahresinzidenzen mehr ausgewiesen. Stattdessen bezieht sich die angegebene Inzidenz (Fälle pro 100.000 Einw.) immer auf den Betrachtungszeitraum gemäß Filtereinstellung. Wird der Betrachtungszeitraum nicht eingeschränkt, unterbleibt also die Auswahl eines oder mehrerer Meldejahre in den Filtereinstellungen, so wird die Inzidenz für den gesamten Zeitraum von 2001 bis Ende des laufenden Jahres angegeben. Bei überjährigen Abfragen ergibt sich die durchschnittliche Jahresinzidenz, indem die ausgewiesene Gesamtinzidenz durch die Anzahl der Jahre im fraglichen Betrachtungszeitraum geteilt wird. Wollen Sie aus einer unterjährigen Abfrage oder für das laufende, noch nicht abgeschlossene Jahr eine Jahresinzidenz hochrechnen, ist insbesondere für Krankheiten mit saisonalen Schwankungen größte Vorsicht geboten.
-
Die zur Inzidenzberechnung herangezogenen Bevölkerungszahlen werden von DESTATIS (www.destatis.de) jeweils zum Stichtag 31.12. bereitgestellt und in SurvStat@RKI 2.0 regelmäßig aktualisiert.
-
Zur jahresübergreifenden Darstellung von Krankheiten mit saisonalem Verlauf kann an Stelle der Meldewoche die sogenannte Saisonwoche als anzuzeigendes Merkmal verwendet werden. Dabei beschreiben die Varianten Saisonwoche (27) und Saisonwoche (40) den Beginn des Saisonjahrs in der 27. Kalenderwoche (Anfang der zweiten Jahreshälfte) und der 40. Kalenderwoche. Für Krankheiten mit minimalen Fallzahlen in der Jahresmitte und maximalen Fallzahlen um den Jahreswechsel herum (z. B. Norovirus-Gastroenteritis) empfiehlt sich die Variante (27); für Influenza und andere Krankheiten, deren Gipfel im 1. Quartal liegt, ist die Variante (40) am besten geeignet. Durch Auswahl definierter Meldewochen in den Filtereinstellungen lässt sich das Saisonjahr auf eine echte Saison eingrenzen (z.B.
Influenzasaison: 01. - 20 KW und 40. - 53.KW
).
-
Referenzdefinition. Die dem Robert Koch-Institut von den Gesundheitsämtern übermittelten Fälle werden vor der Veröffentlichung nach einer Referenzdefinition gefiltert. Alle grafischen Darstellungen und Tabellen in Publikationen des RKI beziehen sich, sofern nicht anders angegeben, auf Fälle, die der Referenzdefinition entsprechen. Dies gilt auch für die Fallzahlen, die in der wöchentlichen Statistik und im Jahresüberblick des Epidemiologischen Bulletins ausgewiesen werden. Zur besseren Vergleichbarkeit mit publizierten Daten sollte auch in Abfragen mit SurvStat@RKI 2.0 die Referenzdefinition berücksichtigt werden, indem die Voreinstellung zum Merkmal Referenzdefinition (= ja) unverändert übernommen wird.
Für Fälle, die direkt an das RKI gemeldet werden, existieren weder Fall- noch Referenzdefinitionen und damit auch keine entsprechenden Auswahlfelder in der Abfragemaske. Die Fallprüfung erfolgt stattdessen im RKI anhand festgelegter Fallkriterien.
-
Bei Abfragen, die den Zeitverlauf einer Krankheit als Histogramm wiedergeben, ist darauf zu achten, dass die Zeitachse keine ‚Lücken‘ aufweist. Wählen Sie dazu insbesondere bei seltenen Krankheiten die Anzeigeoption „Leere Zeilen und Spalten anzeigen“.
-
Abfragen sollten entsprechend der Fragestellung Schritt für Schritt aufgebaut werden (Filtereinstellungen, anzuzeigende Merkmale, Anzeigeoptionen). Um Verwechslungen zu vermeiden und damit Abfrageergebnisse reproduziert werden können, liefert SurvStat@RKI 2.0 mit jedem Download eine Zusammenfassung der Abfrage im PDF-Format mit.
-
Abfrageergebnisse in Form von Tabellen und Abbildungen sollten immer vollständig beschriftet dargestellt werden. Nur so sind sie aussagefähig. Zur vollständigen Beschriftung gehören die dargestellte(n) Krankheit(en), Personen-, Orts- und Zeitangaben sowie der Datenstand.
nach oben
zu einzelnen Krankheiten
-
Für Adenoviren besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht nur für den direkten Nachweis im Konjunktivalabstrich.
-
Beginnend mit März 2014 stehen zur Echinokokkose bis auf Weiteres keine aktuellen Daten zur Verfügung.
-
Die Häufigkeit der Diagnose von enterohämorrhagischen Escherichia coli (EHEC) Infektionen in Deutschland ist sehr von der Inanspruchnahme und Qualität labordiagnostischer Möglichkeiten abhängig. Die Diagnostik dieser Erreger ist schwierig und wird im Routinealltag häufig nicht bis zur Anzucht eines Isolats oder bis zur Bestimmung der Serogruppe durchgeführt, die aber für die epidemiologische Beurteilung erforderlich ist, wie auch der große EHEC-O104-Ausbruch des Jahres 2011 noch einmal verdeutlichte. Da nur für weniger als ein Drittel der EHEC-Fälle Informationen zur Serogruppe vorliegen, haben Angaben zur Serogruppenverteilung in Deutschland nur eine begrenzte Aussagekraft.
-
Für Haemophilus influenzae besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht nur für den direkten Nachweis aus Liquor oder Blut.
-
Seit dem Jahr 2003 wird das enteropathische HUS getrennt von EHEC übermittelt und ausgewertet, da in seltenen Fällen auch andere Erreger enteropathisches HUS hervorrufen können. Für die Jahre 2001 und 2002 waren die HUS-Fälle noch in der Zahl der EHEC-Meldungen enthalten. Dies ist vor allem beim Vergleich der EHEC-Zahlen über mehrere Jahre zu beachten.
-
Für Hepatitis C besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht für alle Nachweise, soweit nicht bekannt ist, dass eine chronische Infektion vorliegt.
-
Für Influenza besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht nur für den direkten Nachweis von Influenzaviren. Seit dem 21.05.2007 wurde die Meldepflicht auf die Meldung gemäß § 6 Abs. 1 Nr. 1 IfSG auf den Krankheitsverdacht, die Erkrankung sowie den Tod eines Menschen an aviärer Influenza ausgedehnt.
-
Bundesweite Daten für die Meldekategorien Keuchhusten, Mumps, Röteln und Windpocken liegen dem RKI erst seit Inkrafttreten der bundesweiten Meldepflicht am 29.03.2013 vor. Bis dahin wurden diese Erkrankungen nur aus den Bundesländern Brandenburg, Mecklenburg-Vorpommern, Sachsen, Sachsen-Anhalt und Thüringen auf der Basis entsprechender Landesverordnungen übermittelt. Neben der Einführung der neuen Meldepflichten wurden 2013 auch die entsprechenden Falldefinitionen aktualisiert. Diese Neuerungen machten Softwareanpassungen für die Datenübermittlung (siehe Abschnitt Datenqualität) notwendig, die in verschiedenen Softwaresystemen z.T. mit erheblicher Verzögerung umgesetzt wurden. Während dieses Zeitraums konnten Fälle oder spezifische Meldesachverhalte entsprechend der neuen Meldepflicht von einigen Gesundheitsämtern nicht übermittelt werden. Das ist bei Datenauswertungen und ihren Interpretationen unbedingt zu berücksichtigen.
-
Ende März 2013 änderte sich auch die gesetzliche Grundlage für die Meldung der konnatalen Röteln von einer nichtnamentlichen Meldung an das RKI gemäß § 7 Abs. 3 IfSG zu einer namentlichen Meldung gemäß § 6 Abs. 1 Nr. 1 und § 7 Abs. 1 IfSG an das zuständige Gesundheitsamt. Auch hier wurde eine neue Falldefinition erarbeitet. Da die Daten für beide Meldewege nur getrennt voneinander abgefragt werden können, lässt sich die Fallzahl seit 2001 nicht in einer gemeinsamen Auswertung/Abbildung darstellen.
-
Für Listeria monocytogenes besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht nur für den direkten Nachweis aus Blut, Liquor oder anderen normalerweise sterilen Substraten sowie aus Abstrichen von Neugeborenen. Seit 01.01.2004 werden Mütter von Neugeborenen mit labordiagnostischem Nachweis von Listeria monocytogenes immer als epidemiologisch-bestätigte Fälle übermittelt, auch wenn es bei der Mutter keinen Labornachweis gibt. Die Fallzahlen in dieser Kategorie ab 2004 sind deshalb nicht direkt mit denen der Jahre vor 2004 vergleichbar.
-
Eine Meldepflicht für invasive Infektionen mit Methicillin-resistentem Staphylococcus aureus (MRSA) besteht erst seit dem 01.07.2009. Die Referenzdefinition erfüllen alle labordiagnostisch nachgewiesenen Infektionen auch bei nicht erfülltem oder unbekanntem klinischen Bild.
-
Für Neisseria meningitidis besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht nur für den direkten Erregernachweis aus Liquor, Blut, hämorrhagischen Hautinfiltraten oder anderen normalerweise sterilen Substraten.
-
Meldepflichtig gemäß § 7 Abs. 1 IfSG ist nur der direkte Nachweis von Noroviren im Stuhl. Seit 2011 erfüllt nur noch die Kategorie der labordiagnostisch bestätigten Norovirus-Erkrankungen die Referenzdefinition. Daher liegen dem RKI keine Informationen zu klinisch-epidemiologisch bestätigten Erkrankungen ohne Labornachweis vor, was zu einer deutlichen Unterschätzung der tatsächlich aufgetretenen Fallzahlen führt. Dies wirkt sich auch auf die Darstellung des zeitlichen Verlaufes, der geografischen Verteilung und der Altersverteilung der Norovirus-Gastroenteritiden aus, da erfahrungsgemäß der Anteil nicht laborbestätigter Fälle im Verlauf des Jahres und zwischen den Bundesländern variiert und bei Ausbrüchen in Abhängigkeit vom Ausbruchsumfeld unterschiedlich hoch ist.
-
Infektionen mit dem enterischen Pathovar von Salmonella Paratyphi B (vormals S. Java) sind in der Kategorie Salmonellose enthalten (siehe "SAL/S.Paratyhi B (enterisches Pathovar, Tartrat positiv, SopE negativ, avrA positiv) - vormals S. Java"). Für Infektionen mit dem systemischen Pathovar von S. Paratyphi B sowie S. Paratyphi A und C (die das Krankheitsbild des Paratyphus hervorrufen), siehe unter Krankheit Paratyphus.
-
Für den Nachweis von Salmonella Typhi und Paratyphi besteht gemäß § 7 Abs. 1 IfSG eine Meldepflicht für alle direkten Erregernachweise.
-
Der Subtyp der monophasischen S. Typhimurium (siehe "SAL / S.Typhimurium, monophasisch") wurde erst im Mai 2012 in die Übermittlungssoftware (SurvNet@RKI; Version 0.8.12) aufgenommen und ist noch nicht in allen Gesundheitsämtern verfügbar. Daher sind Daten zu diesem Salmonellen-Subtyp nicht als Zeitreihe auswertbar.
-
Beginnend mit April 2014 stehen zur konnatalen Toxoplasmose bis auf Weiteres keine aktuellen Daten zur Verfügung.
-
Gemäß § 6 Abs. 1 Nr. 1 IfSG ist nur die Erkrankung sowie der Tod an einer behandlungsbedürftigen Tuberkulose meldepflichtig, gemäß § 7 Abs. 1 IfSG besteht eine Meldepflicht für den direkten Erregernachweis und vorab auch für den Nachweis säurefester Stäbchen im Sputum.
-
Bei Abfragen von Malariafällen ist folgendes zu beachten:
Im Juli 2023 wurde die nichtnamentliche Meldepflicht von Plasmodiennachweisen an das RKI (gemäß § 7 Abs. 3 Infektionsschutzgesetz (IfSG)) in eine namentliche Meldepflicht an das zuständige Gesundheitsamt (gemäß § 7 Abs. 1 IfSG) geändert.
Aus datentechnischen Gründen ist eine Zusammenfassung der auf den beiden Wegen gemeldeten Fälle in einer Abfrage nicht möglich.
– Malariafälle bis einschließlich 2022 finden sich vollständig in einer Abfrage mit dem Meldeweg „Nichtnamentlich direkt an das RKI“.
– Malariafälle ab 2024 finden sich vollständig in einer Abfrage mit dem Meldeweg „Über Gesundheitsamt und Landesstelle“.
– Um die Gesamtzahl der Malaria-Fälle im Jahr 2023 zu ermitteln, müssen die Fälle des Jahres 2023 aus beiden Meldewegen kombiniert werden.
-
Humanpathogene Bornaviren sind seit 2015 (VSBV-1) bzw. 2018 (BoDV-1) bekannt. Der Direktnachweis von humanpathogenen Bornaviren ist seit 1. März 2020 gemäß §7 IfSG Abs. 1 meldepflichtig. Direktnachweise, die vor März 2020 stattgefunden haben, konnten und können weiterhin auf freiwilliger Basis retrospektiv gemeldet werden. Zudem werden humanpathogene Bornaviren z.T. retrospektiv in archivierten Proben (z.B. Liquorbanken, Hirnbanken) nachgewiesen. Meldejahre entsprechen daher nicht den Erkrankungsjahren. Dies betrifft insbesondere die Jahre nach Einführung der Meldepflicht bzw. die Jahre nach Bekanntwerden der Humanpathogenität. Die Meldejahre haben keine Aussagekraft hinsichtlich einer jährlichen Inzidenz und geben tatsächlich nur das Jahr der Meldung wider.
nach oben
zur Datenqualität
-
Elektronische Übermittlung. Das RKI stellt für die Gesundheitsämter und Landesbehörden kostenlos die gemäß den gesetzlichen Vorgaben und Erfordernissen des Meldewesens entwickelte Software SurvNet@RKI zur Verfügung, die zur Erfassung, Auswertung und Übermittlung der Meldedaten gemäß IfSG dient. Die Gesundheitsämter können jedoch auch Softwareprodukte anderer Anbieter für die Bearbeitung und Übermittlung von Meldungen verwenden. Durch den Einsatz der unterschiedlichen Softwareprodukte kann es aufgrund von Kompatibilitätsproblemen auch zur Beeinträchtigung der Datenqualität kommen. Dies kann zu Datenverlust, zur Dopplung von Fällen und zu falsch übermittelten Inhalten führen.
-
Vollzähligkeit. Im Meldesystem können in der Regel nur die Fälle von Infektionskrankheiten erfasst werden, die im medizinischen Versorgungssystem z.B. von Ärzten in niedergelassener Praxis, Krankenhäusern oder Laboratorien erkannt und gemeldet wurden. Wenn infizierte Personen z.B. wegen asymptomatischer oder milder Verläufe keinen Arzt aufsuchen, der Arzt keine Diagnostik in einem Labor veranlasst oder nach Diagnose keine Meldung erfolgt, werden diese Fälle in der Regel nicht im Meldesystem erfasst. Die Untererfassung kann je nach Meldeweg oder Krankheit unterschiedlich stark ausgeprägt sein. Die dargestellten Fallzahlen/Inzidenzen entsprechen demnach nicht allen Neuerkrankungen in der Bevölkerung in einem bestimmten Zeitraum, sondern sind als Fallmeldungen/Meldeinzidenz zu interpretieren.
-
Vollständigkeit. Zu jeder Einzelfallmeldung werden wichtige epidemiologische Informationen erfasst, z.B. Alter, Geschlecht, Angaben zum klinischen Bild und zur Labormaterial und Methode. Diese Informationen liegen bei Meldung häufig nicht vollständig vor und müssen von den Gesundheitsämtern nachermittelt werden. Liegen diese Angaben nicht vor oder können sie nicht nachermittelt werden, werden sie in der Darstellung z.B. nach Altersgruppe oder Geschlecht nicht berücksichtigt.
-
Meldeverzug. Namentliche Meldungen an die Gesundheitsämter werden der Meldewoche, in der das Gesundheitsamt offiziell Kenntnis von einem Fall erlangt, zugeordnet. Bei der Analyse der Meldedaten 2013 hat sich gezeigt, dass im Median 5 Tage zwischen Symptombeginn und Meldedatum vergehen. Bei nichtnamentlichen Meldungen an das RKI wird in der Regel der Diagnosemonat für die zeitliche Zuordnung verwendet.
nach oben
zu Merkmalen
Meldepflichtige Krankheiten und Erregernachweise nach gesetzlicher Grundlage der Meldung. Der Krankheitsbezeichnung vorangestellt ist der zugehörige RKI-Code, wie er in der Auswahl/Anzeige der Erregerangaben verwendet wird.
| RKI-Code |
meldepflichtig gemäß §§ 6.1.1 bzw. 7.1 IfSG |
| ABV | Arbovirus-Erkrankung (seit 2017) |
| ACB | Acinetobacter-Infektion oder -Kolonisation (seit 2017) |
| ADV | Adenovirus-K(eratok)onjunktivitis |
| BAN | Milzbrand |
| BOR | Läuserückfallfieber |
| BOV | Bornavirus-Erkrankung (seit 2023) |
| BPS | Keuchhusten (seit April 2013) |
| BRU | Brucellose |
| CAM | Campylobacter-Enteritis |
| CAU | Candida auris, invasive Infektion (seit 2023) |
| CDF | Clostridioides difficile |
| CHL | Ornithose |
| CJK | CJK (Creutzfeld-Jakob-Krankheit) |
| CJV | neue Variante Creutzfeldt-Jakob-Krankheit |
| CKV | Chikungunya (seit 2017) |
| CLO | Botulismus |
| COR | Diphtherie |
| COX | Q-Fieber |
| CRY | Kryptosporidiose |
| CVD | COVID-19 (seit 2020) |
| CVS | SARS (Schweres Akutes Respiratorisches Syndrom) |
| DEN | Denguefieber |
| EBC | Enterobacterales-Infektion oder -Kolonisation (seit 2017) |
| EBV | Ebolafieber |
| ECO | E.-coli-Enteritis |
| EHC | EHEC-Erkrankung |
| FRT | Tularämie |
| FSV | FSME (Frühsommer-Meningoenzephalitis) |
| GFV | Gelbfieber |
| GIL | Giardiasis |
| HAV | Hepatitis A |
| HBV | Hepatitis B |
| HCV | Hepatitis C |
| HDV | Hepatitis D |
| HEV | Hepatitis E |
| HFA | Virale Hämorrhagische Fieber |
| HIN | Haemophilus influenzae, invasive Erkrankung |
| HTV | Hantavirus-Erkrankung |
| HUS | HUS (Hämolytisch-urämisches Syndrom), enteropatisch |
| HXV | Hepatitis Non A-E |
| INV | Influenza |
| LEG | Legionellose |
| LEP | Leptospirose |
| LIS | Listeriose |
| LSV | Lassafieber |
| MBV | Marburgfieber |
| MPV | Mumps (seit April 2013) |
| MPX | Mpox (seit 2022) |
| MRA | MRSA (Methicillin-resistenter Staphylococcus aureus), invasive Infektion (seit Juli 2009) |
| MSV | Masern |
| MYL | Lepra |
| MYT | Tuberkulose |
| NCV | Nicht-Cholera-Vibrionen-Erkrankung (seit 2023) |
| NEI | Meningokokken, invasive Erkrankung |
| NOV | Norovirus-Gastroenteritis |
| OPX | Orthopocken (seit 2023) |
| PKV | Pocken |
| PLA | Malaria (seit 2024) |
| POV | Poliomyelitis |
| RBV | Tollwut |
| RIC | Fleckfieber |
| RSV | RSV-Infektion (seit 2023) |
| RTV | Rotavirus-Gastroenteritis |
| RUN | Röteln, konnatale Infektion (ab April 2013) |
| RUV | Röteln, postnatal (seit April 2013) |
| SAL | Salmonellose |
| SHI | Shigellose |
| SPA | Paratyphus |
| SPN | Pneumokokken, invasive Erkrankung (seit 2020) |
| SSP | Subakute Sklerosierende Panenzephalitis (seit 2023) |
| STY | Typhus abdominalis |
| TRI | Trichinellose |
| VCH | Cholera |
| VZV | Windpocken (seit April 2013) |
| WNV | West-Nil-Fieber (seit 2023) |
| YEN | Yersiniose |
| YPS | Pest |
| ZKV | Zikavirus-Erkrankung (seit 2016) |
nach oben
| RKI-Code |
meldepflichtig gemäß 7.3 IfSG |
| ECH | Echinokokkose |
| HIV | HIV-Infektion |
| PLA | Malaria (bis 2023) |
| RUN | Röteln, konnatal (bis März 2013) |
| TOX | Toxoplasmose, konnatal |
| TRP | Syphilis |
nach oben
| RKI-Code |
meldepflichtig gemäß länderspezifischer Meldeverordnung |
| AD2 | Adenovirus, Länderverordnung (ST) |
| BOB | Borreliose (BY, BE, BB, MV, RP, SL, SN, ST, TH) |
| CLT | Tetanus (SN) |
| EAH | Amoebiasis (MV, SN, TH) |
| GBR | Gasbrand (SN) |
| MGV | Meningoenzephalitis, viral (SN, ST, TH) |
| SPN | Pneumokokken, invasive Erkrankung (MV, SN, ST, TH) |
| SPY | Scharlach (SN, TH) |
| TO2 | Toxoplasmose, postnatal (SN) |
| TTV | Tollwutexpositionsverdacht (ST) |
nach oben
Bundesländer (Abkürzung in Klammern) und zugehörige Gebietseinheiten (NUTS Ebene2)
- Baden-Württemberg (BW)
- Stuttgart
- Karlsruhe
- Freiburg
- Tübingen
- Bayern (BY)
- Oberbayern
- Niederbayern
- Oberpfalz
- Oberfranken
- Mittelfranken
- Unterfranken
- Schwaben
- Berlin (BE)
- Berlin
- Brandenburg (BB)
- Brandenburg (keine Unterteilung in Brandenburg - Nordost und Brandenburg - Südwest)
- Bremen (HB)
- Bremen
- Hamburg (HH)
- Hamburg
- Hessen (HE)
- Darmstadt
- Gießen
- Kassel
- Mecklenburg-Vorpommern (MV)
- Mecklenburg-Vorpommern
- Niedersachsen (NI)
- Braunschweig
- Hannover
- Lüneburg
- Weser-Ems
- Nordrhein-Westfalen (NW)
- Düsseldorf
- Köln
- Münster
- Detmold
- Arnsberg
- Rheinland-Pfalz (RP)
- Koblenz
- Trier
- Rheinhessen-Pfalz
- Saarland (SL)
- Saarland
- Sachsen (SN)
- Dresden
- Chemnitz
- Leipzig
- Sachsen-Anhalt (ST)
- Sachsen-Anhalt
- Schleswig-Holstein (SH)
- Schleswig-Holstein
- Thüringen (TH)
- Thüringen
nach oben